usp class vi vs fda
The USP-FDA relationship dates back to the 1906 Pure Food and Drug Act which deemed the United States Pharmacopeia and the National Formulary. USP stands for US.

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
USP-FDA Shared History and Mission.

. Among all USP classes Class VI materials meet the most stringent testing requirements. It consists of 3 testing. These standards define the purity and reliability of a product or process and prioritize public wellbeing.
Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. Typical applications for our FDA NSF. KTW FDA USP Class VI EB152-70 3407 General Purpose -70 to 250.
27 rows The USP Class VI compounds must be made from ingredients with clear histories of. Most applications are fairly benign. Moulded O-rings class 1 less than 10 furnace black These can be produced in all.
Specially formulated for long term sealing. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. When evaluating a new product many of our.
Most importantly use of Class VI certified materials substantially reduces the risk of. USP Class VI Chapter 88 relates to in vivo biological reactivity tests its purpose is to determine the biological response impact of elastomeric materials on live animals. Sil 714001 USP class VI Silicone 1 70 Yes transl.
USP Class VI approval tests are one of the important standards set. The United States Pharmacopeia USP is a non-governmental not-for-profit public health organization that is an official public standards-setting authority for all. Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and.
Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being. There may be some confusion between FDA USP Class VI and FDA food grade materials. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600.
FDA and USP Class VI materials are available in all standard o-ring dimensions AS568 custom o-ring sizes and specialty molded products. USP Plastic Class VI as this group is also known includes silicones that have. It generally ensures a high quality level and better acceptance with the FDA and USDA.
USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. FDA food-grade rubber materials typically comply with FDA 21. Class VI materials which were discussed earlier are tested according to the above protocols.
Sil 714002 USP class VI Silicone 1 70 Yes transl.

The Value Of Usp Class Vi Testing For Medical Device Cable And Wire Medical Design Briefs

O Ring Epdm 70 Black Peroxide Fda Ec 1935 2004 Kiwa Usp Class Vi 3 A Sanitary Standard Nsf 61 Nsf 51 Wras Dvgw Easy To Order At Sealsupply Eu
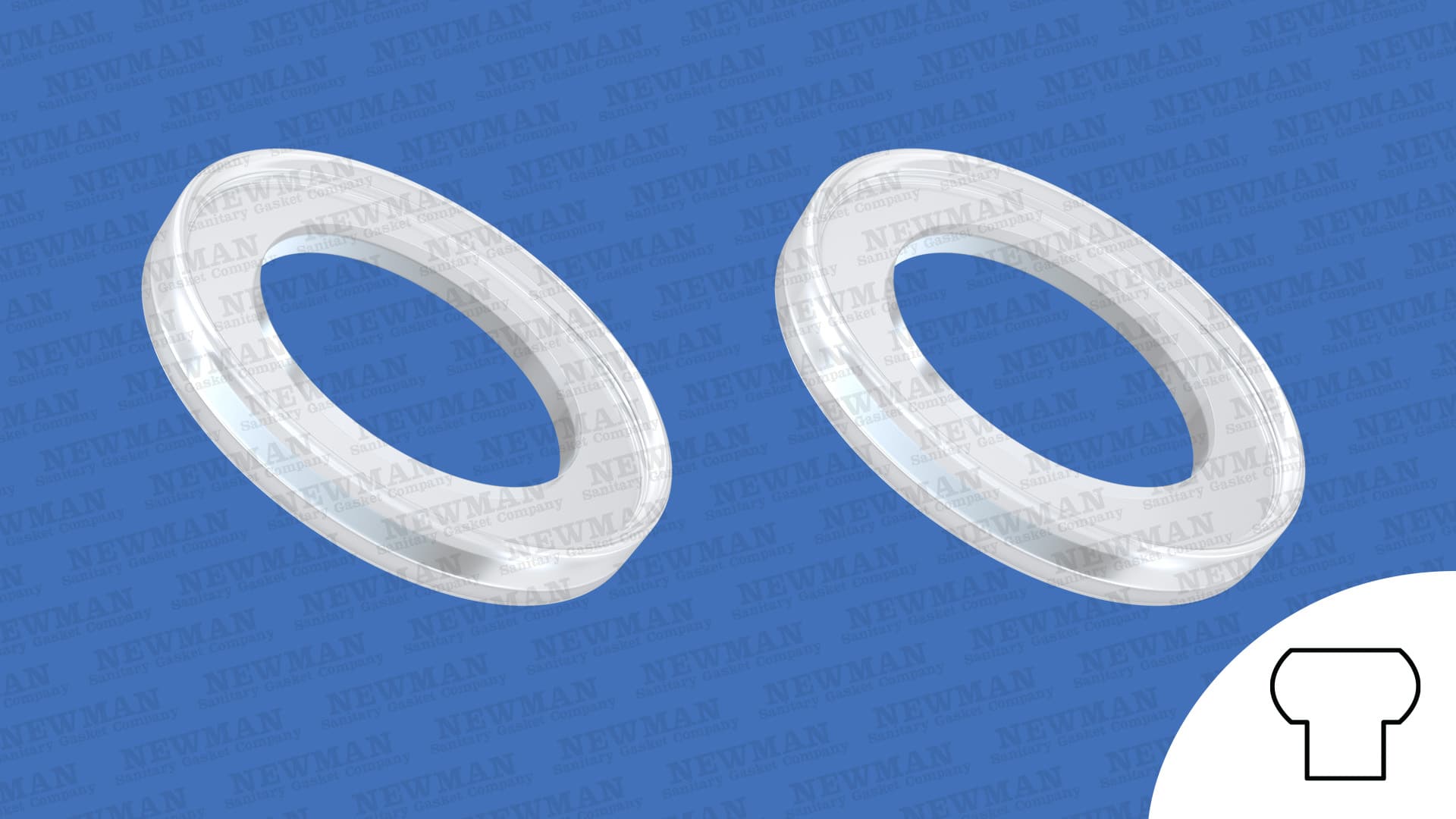
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

China Fda Usp Class Vi Ffkm Hygiene Sanitary Compounds Photos Pictures Made In China Com

Vena Sil 650v Silicon Hose Fda Bfr Usp Class Vi Lazaro Id

Usp Class Vi Foster Corporation

Usp Class Vi Medical Grade Plastic Materials Order Online

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Silicone Tubing Working Temperature 60 C To 200 C Fda Usp Class Vi A 50 60 Lp Italiana

T Series Usp Class Vi Fda Hoses Ace Sanitary

T Series Usp Class Vi Fda Hoses Ace Sanitary
Medical Die Cutting Custom Cut Fda Approved Materials

Usp Class Vi Adi Free What Does It All Mean

Pharmaceutical And Cosmetics Production


